According to the National Medical Products Administration, China recently granted provisional approval to Shanghai Wangshi Biomedical Technology Co., Ltd.'s application to administer oral antiviral treatments for COVID-19, also known as VV116.
VV116 is an oral small-molecule drug, which is effective against coronavirus and used for the treatment of mild and moderate COVID-19 adult patients. Medical advice must be followed at all times.
Based on positive clinical trial results, VV116 was approved to be used in the treatment of moderate to severe COVID-19 patients in Uzbekistan in late 2021.
The approval of VV116 will play a critical role in reducing the COVID-19 threat to people's health and in China's COVID-19 prevention and control system.
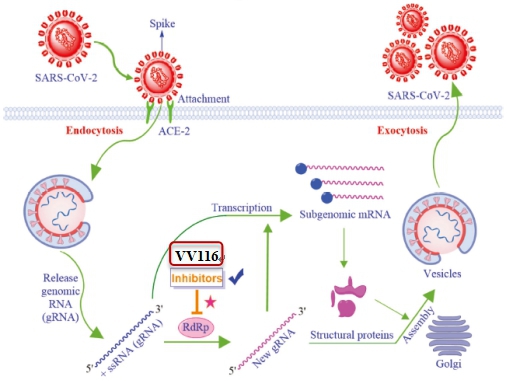
VV116: Mechanism of Action.
MORE ABOUT VV116
VV116 vs Paxlovid: comparable efficacy
In a phase 3, noninferiority, observer-blinded, randomized trial of VV116 versus Paxlovid published in The New England Journal of Medicine (NEJM), symptomatic adults with mild-to-moderate COVID-19 with a high risk of progression were assigned to receive a 5-day course of either VV116 or nirmatrelvir-ritonavir.
In the final analysis, the time to sustained symptom resolution and the first negative SARS-CoV-2 test did not differ substantially between the two groups.
VV116 vs Paxlovid: VV116 has fewer adverse reactions
Through 28 days of follow-up, participants who received VV116 reported fewer adverse events than those who received nirmatrelvir-ritonavir (67.4% vs. 77.3%), as well as fewer grade 3 or 4 adverse events (2.6% vs. 5.7%) .
Unlike nirmatrelvir-ritonavir, which has drug interactions with multiple medications, VV116's interaction with concomitant medications is less likely.
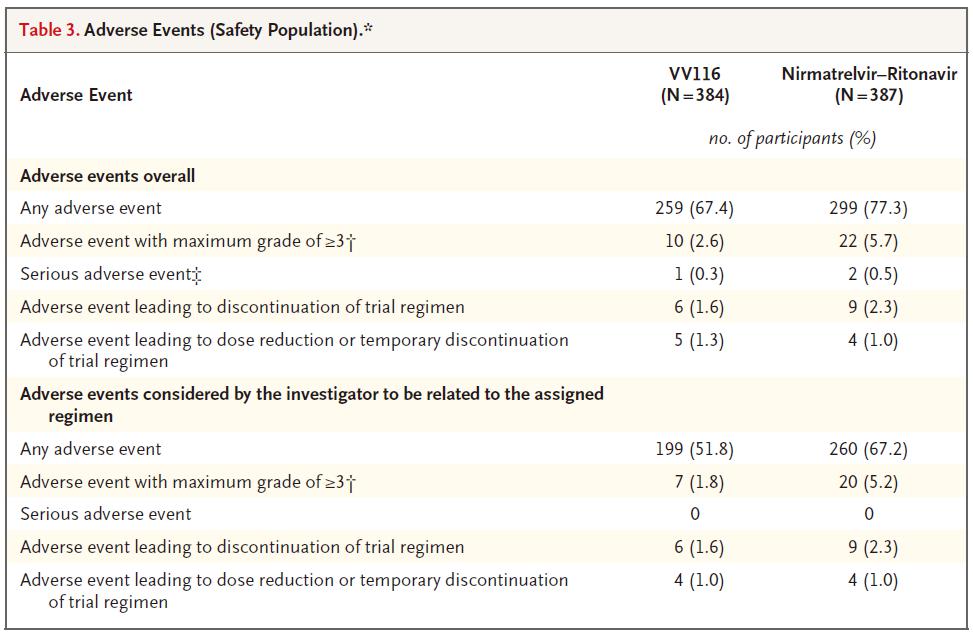
VV116 vs Paxlovid: Adverse Events.
Author | Hannah, Riz Zhang (intern)
Editor | Wing, Nan, Monica, James
Source | The Sixth Affiliated Hospital, Sun Yat-sen University
















